FDA document reveals 86% of children who participated in Pfizer covid vaccine trial experienced adverse reactions
05/27/2021 / By Mike Adams

A publicly-available FDA “fact sheet” document reveals that 86% of children who participated in a Pfizer covid vaccine trial reported adverse reactions ranging from “mild” to “serious.”
As part of the vaccine experiments, children aged 12 to 15 are being injected with mRNA sequences that take control of their cells, causing them to churn our spike proteins in their blood. Spike proteins cause vascular disease and blood clots. Even the Jonas Salk Institute conclusively identifies spike proteins as the culprit behind vascular disease and blood clots.
This is all openly admitted by the FDA, which has published extremely disturbing reports of adverse reactions experienced by children in a Pfizer covid vaccine “fact sheet” labeled 144413. See the original FDA document here (PDF).
In case the FDA removes this sheet, we have archived it at Natural News servers here (PDF).
FDA admits mRNA vaccines cause adverse reactions in 86% of children, but calls it “safe” anyway
This Pfizer page at the FDA provides links to all the fact sheets and press releases where the FDA celebrates expanding its emergency use authorization to children aged 12 to 15.
That fact sheet contains the following table that details the alarming rate of side effects and damage experienced by 12 – 15 year olds (i.e. children) who were given the mRNA injections:
Table 5: Study 2 – Frequency and Percentages of Adolescents With Solicited Local Reactions, by Maximum Severity, Within 7 Days After Each Dose – Adolescents 12 Through 15 Years of Age
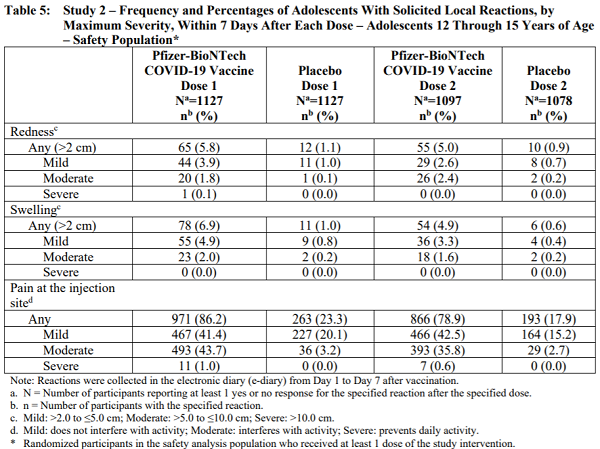
As you can see from the table, 1127 children were given the first dose of the vaccine, and 1097 children received the second dose. What happened to the 30 children who didn’t show up for the second dose? Did they die? Why were they removed from the second dose?
Among those children injected with the mRNA vaccine medical experiment:
- A shocking 86% experienced side effects.
- Nearly 44% suffered “moderate” side effects defined as “interfering with activity.”
- 66% of the children experienced fever.
- 65% suffered headaches.
- Other side effects experienced by these children as part of these medical experiments include chills, vomiting, diarrhea, fever, muscle pain and even joint pain.
- Even after 86% of children experienced such side effects after being injected with the first dose, researchers continued to inject the children with a second dose.
The FDA claims this is all about “protecting” children while pushing more vaccine sales to generate billions of dollars in profits for Pfizer
The FDA claims in its celebratory press release that expanding Pfizer’s experimental vaccine to 12 to 15-year-olds is a kind of breakthrough, not a crime against children:
“The FDA’s expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age is a significant step in the fight against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, M.D. “Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic. Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
In other words, the FDA is admitting they are fully aware of the 86% side effect rate when it comes to injecting children with experimental mRNA “vaccines.”
Vaccine medical experiments are harming children in the name of Big Pharma profits
When it comes to mRNA vaccine trials in the USA, when serious reactions such as facial paralysis are identified in the vaccinated group, the FDA (and presumably, the researchers) dismiss them as coincidence. From the FDA’s own document:
Bell’s palsy (facial paralysis) was reported by four participants in the Pfizer-BioNTech COVID-19 Vaccine group. Onset of facial paralysis was Day 37 after Dose 1 (participant did not receive Dose 2) and Days 3, 9, and 48 after Dose 2. No cases of Bell’s palsy were reported in the placebo group. Currently available information is insufficient to determine a causal relationship with the vaccine.
Thus, no matter what horrific side effects are caused by the vaccine experiment, they are dismissed and ignored. After all, there are billions of dollars to be earned from authorizing vaccines for widespread use in children. (It’s a whole new demographic market to target.)
This FDA document even admits the vaccine is not approved and may cause serious injury or even death:
FDA has authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine, which is not an FDA-approved vaccine.
Adverse Reactions in Clinical Trials
Adverse reactions following the Pfizer-BioNTech COVID-19 Vaccine that have been reported in clinical trials include injection site pain, fatigue, headache, muscle pain, chills, joint pain, fever, injection site swelling, injection site redness, nausea, malaise, and lymphadenopathy (see Full EUA Prescribing Information).
Adverse Reactions in Post Authorization Experience
Severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema), diarrhea, vomiting, and pain in extremity (arm) have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine outside of clinical trials. Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine.
The FDA also admits that life-threatening anaphylactic shock may occur following the vaccine, or that vaccine recipients may lose consciousness:
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine.
Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting.
Any rational person, after reading this “fact sheet” from the FDA, would express serious concern over the continued recruitment and exploitation of children as human guinea pigs in vaccine medical experiments.
This is why we continue to sound the alarm on such practices.
Submit a correction >>
Tagged Under:
adverse reactions, covid vaccines, FDA, immunity, immunizations, pandemic, Pfizer, side effects, vaccine wars
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
Infections.News is a fact-based public education website published by Infections News Features, LLC.
All content copyright © 2018 by Infections News Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.